 Get premium membership
Get premium membership and access revision papers with marking schemes, video lessons and live classes.
a).(i) Name the solution and the catalyst used in preparation of oxygen in the laboratory.
(ii) Give a chemical equation for the reaction above.
(b) In an experiment to determine the proportion of oxygen in air, Copper turning were packed in excess in a combustion tube connected to two syringes of 80cm3 each in a volume . Syringe R contained 80cm3 of air while syringe S was closed and empty as shown.
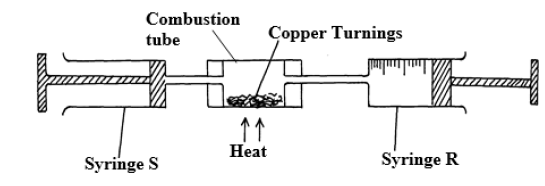
Air was passed over heated turnings slowly and repeatedly until there was no further
change in volume. After cooling, 63.2cm3 of air remained in syringe R.
Why was copper packed in excess?
ii).State one observation made in the combustion tube during experiment.
iii).Give an equation for the reaction that took place in combustion tube.
(iv) Determine the percentage of oxygen used up during the experiment.
(c) Study the diagram below and answer the questions that......